A new standard of care where CAR-T manufacturing is reliably completed in 2.5 days
Trenchant aims to make life-saving CAR-T therapies truly accessible to patients by changing the current CAR-T manufacturing approach.
Our team of scientists and engineers have designed and are currently testing an automated end-to-end platform that can perform the entire CAR-T manufacturing process in 2.5 days to make it more accessible and affordable to patients.
Follow Trenchant’s journey as we make manufacturing CAR-T in 2.5 days a reality by joining our email list
Our Approach
Our automated and truly end to end manufacturing platform starts with apheresis or whole blood, and ends with a cell therapy dose 2.5 days later
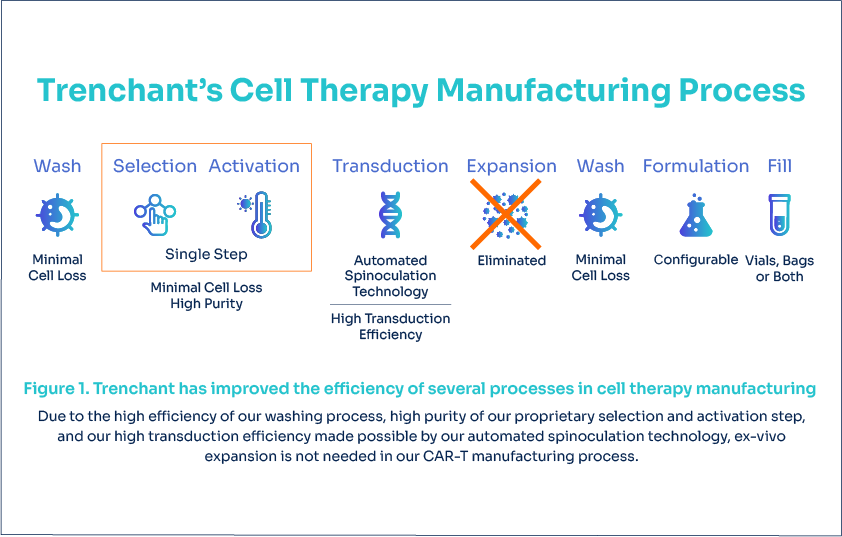
Trenchant has optimized several key processes in CAR-T manufacturing (Figure 1) and thereby significantly minimized cell losses. The final output is a drug product dosed into either bags, vials, or both depending on user requirements. Our process includes the following highly efficient technologies:
Washing
Proprietary Selection & Activation
Transduction
Formulation and Fill technologies
It’s so efficient, there is no need for cell expansion in our process.
All processes performed in a single cell processing cassette
Trenchant’s automated platform contains all processes within one cassette, further reducing cell loss and risk that occurs when moving cells from platform to platform. The combination of this and Trenchant’s optimized processes greatly reduce cell loss that is currently too common in CAR-T manufacturing.
Trenchant’s automated platform eliminates the need for ex-vivo expansion
Current manufacturing processes typically suffer significant cell loss. Ex-vivo expansion is necessary to achieve the required clinical cell dose, and can be problematic for highly pre-treated patient populations.
Trenchant’s highly efficient wash, selection, activation and transduction technologies drastically reduces the number of cells lost, thus eliminating the need for ex-vivo expansion (Figure 1).